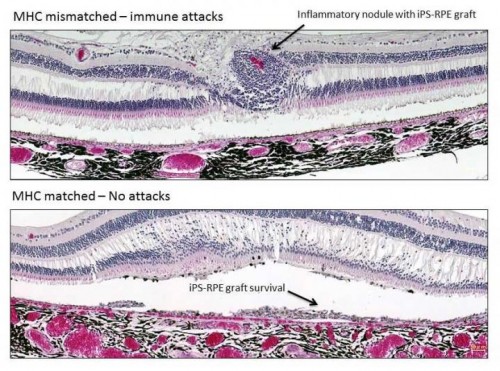
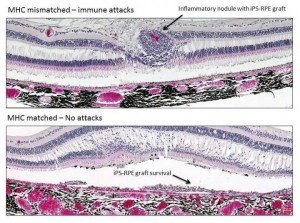
A new treatment that prevents retinal transplant rejection using immune-matched stem cells may be used to treat age-related macular degeneration and other retinal diseases.
Stem cell-based transplantation approaches hold great potential for treating a wide range of eye diseases, but progress has been limited by concerns about cost, safety, and effectiveness.
In two related studies published Sept. 15 in Stem Cell Reports, the journal of the International Society for Stem Cell Research (ISSCR), scientists in Japan overcame a part of these concerns by demonstrating the successful transplantation of stem cell-derived retinal cells generated from immunologically matched donor animals without the need for harmful immunosuppressants.
“Our findings address a major controversy in the stem cell transplantation field by showing that retinal cell grafts are attacked by the immune system if the donor and recipient are not immune-matched, and that matching prevents immune attacks against the grafts without the need for immunosuppressants,” says first author Sunao Sugita of the RIKEN Center for Developmental Biology. “This approach could potentially be used to treat age-related macular degeneration and other retinal diseases in humans.”
In recent years, induced pluripotent stem cells (iPSCs) have generated a great deal of interest as a potentially unlimited source of various cell types for transplantation. This approach involves genetically reprogramming skin cells taken from adult donors to an embryonic stem cell-like state and then converting these immature cells into specialized cell types found in different parts of the body.
In a procedure called autologous cell replacement therapy, iPSC-derived cells generated from a patient are transplanted back into that same patient’s own body, thereby minimizing the potential for graft rejection by the immune system. However, this approach is costly and not suitable for patients who need grafts immediately.
Continue Reading Below ↓↓↓
An attractive alternative is allogeneic cell replacement therapy, which involves transplanting iPSC-derived cells generated from one patient into a different patient’s body. Although this strategy is less costly and more widely applicable, it raises concerns about the potential for immune rejection of grafts and the need for immunosuppressants, which can increase the risk of cancer and serious infections.
There has been an ongoing controversy as to whether the safety and effectiveness of allogeneic cell replacement therapy could be improved through the use of iPSC-derived cells generated from immunologically matched donors.
In the new study, Sugita and senior author Masayo Takahashi, who leads the Laboratory for Retinal Regeneration at RIKEN, directly addressed this controversy, focusing on retinal pigment epithelial cells.
The researchers transplanted iPSC-derived retinal pigment epithelial cells generated from donor monkeys into the eyes of recipient monkeys.
In some cases, the donors and recipients were immunologically matched, that is, their cells expressed the same major histocompatibility complex (MHC) proteins; in other cases, the grafts were mismatched, signaling to the immune system that a foreign substance was present in the body.
Transplantation of MHC-mismatched grafts produced retinal tissue damage and clear signs of immune rejection. In contrast, MHC-matched grafts produced no signs of immune rejection and survived until the final evaluation six months after the procedure, even though no immunosuppressants were used.
“I was surprised that the immune system of recipients rejected grafts from mismatched donors, since retinal pigment epithelial cells are able to suppress the activation of inflammatory cells,” Sugita says. “However, our results clearly demonstrate that the transplanted grafts do not survive because of the immune attacks.”
In a related study, the researchers provided a molecular explanation for these findings. Human iPSC-derived retinal pigment epithelial cells activate MHC-mismatched human T cells, but not immunologically matched human T cells.
One major limitation of the new research is that the monkeys used were not disease models. The researchers are currently developing animal models of age-related macular degeneration to determine how they respond to transplantation of iPSC-derived cells from matched donors.
In addition to preclinical studies, the researchers are planning clinical protocols for the transplantation of iPSC-derived retinal pigment epithelial cells in MHC-matched patients with age-related macular degeneration. “We are hopeful that this approach will prevent immune rejection, prolong graft survival, and improve quality of life for many patients with ocular diseases,” Sugita says.
Continue Reading Below ↓↓↓
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Charitable Trust Fund for Ophthalmic Research; the Project for Realization of Regenerative Medicine from MEXT; and by the Research Center Network for Realization of Regenerative Medicine from the Japan Agency for Medical Research and Development, AMED.
Stem Cell Reports, Sugita et al.: “Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models” http://www.cell.com/stem-cell-reports/fulltext/S2213-6711(16)30177-1 / http://dx.doi.org/10.1016/j.stemcr.2016.08.010
Stem Cell Reports, Sugita et al.: “Lack of T Cell Response to iPSC-Derived Retinal Pigment Epithelial Cells from HLA Homozygous Donors” http://www.cell.com/stem-cell-reports/fulltext/S2213-6711(16)30178-3 / http://dx.doi.org/10.1016/j.stemcr.2016.08.011
Source: Cell Press
Journal: Stem Cell Reports
Photo Credit: Sunao Sugita, MD, PhD