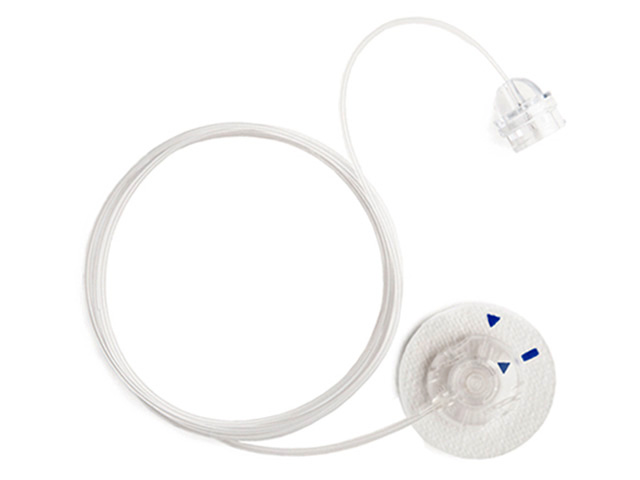
Medtronic plc announced that it has started to inform patients worldwide of a voluntary recall of specific lots of infusion sets used with all models of Medtronic insulin pumps.
The recall is related to a certain discontinued component in these infusion sets and does not include insulin pumps or glucose sensors.
The company determined, through recent field reports from patients and root cause analysis, that a component, the vent membrane, in the recalled infusion sets may be susceptible to being blocked by fluid during the process of priming/fill-tubing. This situation can lead to potential over-delivery of insulin shortly after an infusion set change, which may cause hypoglycemia.
Currently manufactured infusion sets, available to patients since April 2017, include a design update of this component which the company believes reduces the risk of insulin over-delivery after an infusion set change.
The company will work with patients to ensure recalled infusion sets with the discontinued component are returned and replaced with new infusion sets containing the updated component at no cost.
Medtronic has contacted the U.S. Food and Drug Administration (FDA), along with other regulatory bodies around the world, to share information related to this issue. Medtronic will continue working directly with government and regulatory authorities on this global voluntary recall.
Continue Reading Below ↓↓↓
“Our priority is to work with our patients to mitigate risk to patient safety. While we have shipped a significant number of the new and enhanced sets since April, we are committed to replacing recalled infusion sets for all patients,” said Francine Kaufman, M.D., chief medical officer of the Diabetes Group at Medtronic. “Our Medtronic Diabetes team will work as quickly as possible to complete all exchanges to the new and enhanced set and fully support our customers throughout this process.”
Customer Instructions
Medtronic recommends that customers use only infusion sets made with the new and enhanced component, the membrane, starting with their next set change. Medtronic would like to remind customers that it is very important to carefully follow the Key Steps document included with the recall notification letter regarding the priming/fill-tubing process – especially if a person only has recalled infusion sets.
Go to https://checklots.medtronicdiabetes.com to determine if you have recalled infusion sets. You will be prompted to enter the REF and LOT numbers for all infusion sets in your possession. The website will then tell you which infusion sets are part of this recall and which are not.
Your REF and LOT numbers are listed on the labels as shown in the examples below:
Box Label

Individual Infusion Set Label

Continue Reading Below ↓↓↓
Customers in the United States (U.S.) can determine if they have recalled infusion sets by visiting https://checklots.medtronicdiabetes.com.
See a copy of the U.S. recall notification letter here: U.S. Medtronic recall notification letter
See priming/fill-tubing instructions here: Medtronic priming/fill-tubing instructions
See more information on infusion sets here: Medtronic infusion sets
Customers outside the U.S. will receive instructions specific to their country.
In Europe, Middle East and Africa (EMEA region), customers can determine if they have recalled infusion sets by visiting: Medtronic EMEA region page
Patients can always consult the advice of their healthcare professional regarding their medical treatment. If a customer in the U.S. has experienced an issue with the use of a Medtronic infusion set, please report it to the 24-hour helpline at +1-800-204-7616. Customers can also report adverse events to the FDA’s MedWatch Adverse Event Reporting program (+1-800-FDA-1088)