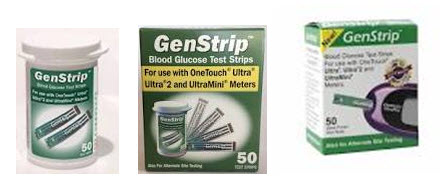
GenStrip Blood Glucose Test Strips, which are advertised for use with the LifeScan OneTouch family of glucose meters, may report false results. Find out which strips are affected.
Audience:
- People with type I and type II diabetes who monitor their blood sugar (blood glucose) levels
- Health Care Professionals including pharmacists, clinicians and health educators who provide diabetes care and management
- Pharmacy retailers and wholesalers
- Hospital Supply Managers
Medical Specialties: Diabetes Care and Management, Pharmacy
Product:
GenStrip Blood Glucose Test Strips, sold by Shasta Technologies LLC, are “third-party” blood glucose monitoring test strips—this means that the test strips are not made by the same company as the meter with which they are used. The strips are used in the home and in health care facilities to measure blood glucose levels in diabetes care and management. Shasta’s GenStrips are advertised for use with the LifeScan OneTouch family of glucose meters (e.g. Ultra, Ultra 2 and Ultra Mini).
The affected test strips have been manufactured and distributed since March 2013 and are available through online retailers and retail pharmacies.
Purpose:
The FDA is advising people with diabetes and health care professionals to stop using GenStrip Blood Glucose Test Strips because the strips may report incorrect blood glucose levels. The FDA recommends the use of alternative glucose test strips that are designed for use with the LifeScan OneTouch family of glucose meters.
Summary of Problem and Scope:
During a recent inspection of Shasta Technologies LLC, the FDA found extensive violations of federal regulations intended to assure the quality of products in the manufacturing of GenStrip Test Strips. These regulations, called quality systems regulations, require manufacturers to establish and document procedures that assure quality, including ways to deal with customer complaints, adverse event reports, and purchasing from suppliers. These regulations also require manufacturers to establish and document procedures for assuring the quality of manufactured product, and for investigating and correcting manufacturing problems.
Continue Reading Below ↓↓↓
At an inspection earlier this year, and documented in an April 2014 warning letter, the FDA found that Shasta Technologies did not have in place many of the requirements of a quality system. Without assurance of an adequate quality system, the FDA believes that the strips could report incorrect blood glucose levels.
People with diabetes carefully monitor their blood glucose levels and require urgent treatment for low blood sugar (hypoglycemia) or high blood sugar (hyperglycemia). An inaccurate blood glucose reading could lead to inappropriate or delayed treatment that could significantly harm a patient.
To date, the company has been unwilling to voluntarily recall their test strips, resulting in their continued availability. The FDA recommends that use of Shasta Technologies, LLC GenStrip Test Strips be discontinued.
The FDA has cleared other glucose test strips that are designed for use with the LifeScan OneTouch family of glucose meters. The FDA urges customers to use these alternatives with their LifeScan OneTouch glucose meters.
Recommendations:
Identify whether you are using GenStrips glucose test strips. The strips may be packaged in green and white packaging with the GenStrip name on top, similar to those shown below.

For People with Diabetes:
- Discontinue use of GenStrip Blood Glucose Test Strips.
- Obtain alternative glucose test strips that are designed for use with the LifeScan OneTouch family of glucose meters.
- Ask your pharmacist or contact your diabetes care provider if you need help determining which test strips to use with your glucose meter.
- As always, be aware of symptoms of high blood sugar (hyperglycemia) and low blood sugar (hypoglycemia). If you experience symptoms of either high or low blood sugar, contact your diabetes care provider for advice on treatment.
For Health Care Providers:
- Discontinue use of GenStrip Blood Glucose Test Strips.
- Obtain alternative glucose test strips that are designed for use with the LifeScan OneTouch family of glucose meters.
For Distributors/Retailers:
- Discontinue sale and distribution of GenStrip Glucose Test Strips.
- Remove unsold product from shelves.
FDA Activities:
On April 8, 2014, the FDA sent a Warning Letter to Shasta Technologies LLC for violations of Federal Quality Systems Regulations.
Continue Reading Below ↓↓↓
Reporting Problems to the FDA:
Prompt reporting of adverse events can help the FDA identify and better understand the risks associated with medical devices.
If you suspect or experience a problem with GenStrip Blood Glucose Test Strips, we encourage you to file a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting program. Health care personnel employed by facilities that are subject to FDA’s user facility reporting requirements should follow the reporting procedures established by their facilities.
Date Issued: April 29, 2014