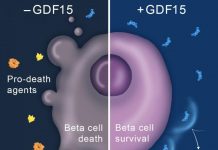
Researchers at the Stanford University School of Medicine have identified a key molecular pathway responsible for the natural decrease in the proliferation of insulin-producing cells that occurs as a person ages. Artificially activating this pathway, which is normally not functional in adults, may be a new way to combat diabetes.
"We're hopeful that soon we might be able to manipulate this pathway in a therapeutic way in humans," said professor of developmental biology Seung Kim, MD, PhD, "perhaps by rekindling its expression and then activating it through a drug we could give in an injection or through some other route. This could be a kind of one-two punch against diabetes."
Kim, who is also a Howard Hughes Medical Institute investigator, is the senior author of the research, which will be published online Oct. 12 in Nature. Research associate Hainan Chen, PhD, is the first author.
The researchers found that, in mice and humans, the pathway is governed by the expression of a molecule called platelet-derived growth factor receptor. PDGF-receptor expression declines over time in mice and humans in a pattern that parallels the decrease in the proliferation of pancreatic beta cells, which produce insulin to control blood sugar levels.
Beta cells are found in the islets of the pancreas. They are the only cells in the body that produce insulin, a hormone that signals the body to remove sugar from the blood after a meal and store it in a variety of cells. Without adequate insulin production, blood sugar levels can become dangerously high -- a condition called hyperglycemia -- and cause organ damage or even coma and death. Type-1 diabetes is caused by a failure to produce insulin; type-2 diabetes is caused by combined deficits in the body to respond to and make insulin. Both types have been linked to reductions of insulin-producing beta cells.
Continue Reading Below ↓↓↓
Kim's experiments in mice showed that artificially activating the PDGF-receptor pathway increases the number of pancreatic beta cells in the animals without compromising their ability to appropriately control their blood sugar levels. The finding is important because other treatments previously shown to stimulate beta cell growth have led to excess insulin production and dangerous hypoglycemia.
It's been known for some time that beta cell proliferation in the pancreas, which is robust in newborn and young animals, decreases dramatically with age. The expression of one molecule known to be involved, Ezh2, declines over time in a similar manner. However it was not known what controlled the changes in Ezh2 expression levels in beta cells.
Because PDGF signaling regulates the proliferation of many types of cells, and is known to affect Ezh2 responses, Kim and Chen wondered if the PDGF pathway was involved in changes in Ezh2 expression and beta cell proliferation. They found that the expression of PDGF receptors was also reduced in pancreatic islet cells from juvenile mice in a pattern similar to the reduction in beta cell proliferation.
When the researchers blocked the expression of PDGF receptors in laboratory mice, they found that young animals (2 to 3 weeks old) made less Ezh2 and had significantly fewer beta cells than control animals. They also had slightly elevated blood sugar levels and were less effective than control animals in disposing of blood sugar when challenged with high glucose. Adult animals lacking expression of the beta cell PDGF receptor were also less able than their peers to regenerate beta cells that were artificially damaged by a chemical compound, and they became severely diabetic after such treatment.
In contrast, islet PDGF receptor expression was increased in normal mice treated with the same compound, and the animals were able to replace the damaged beta cells within about three to four weeks. Although those animals did become moderately diabetic after treatment, they regained control of their blood sugar levels after the cells were regenerated.
"When we destroyed the beta cells with this toxin, the expression of PDGF receptor was induced," said Kim, "and the beta cells naturally recovered. Crucially, the cells didn't go crazy; they underwent only modest, appropriate proliferation. This is good, because it means the beta cells remained in control, and didn't lose critical functions while growing."
When the researchers added the PDGF protein (which binds to and activates the PGDF receptor) to islet cells grown in the laboratory, they found that beta cells in islets from young (3-week-old) mice began to proliferate. In contrast, those from adult (7- to 9-month-old) mice did not respond � because they no longer express the PDGF receptor.
The researchers then created a strain of laboratory mice in which the PDGF receptor was always active in beta cells. When these mice were 14 months old (middle-age for mice), the rate of beta cell proliferation was nine times that of age-matched controls. Despite the increased rate of growth, however, the mice continued to regulate their blood sugar levels appropriately.
In what is arguably the most exciting aspect of their work, Chen and Kim investigated if a similar pathway is functioning in human beta cells. To do so, they obtained pancreatic islets from young human organ donors between the ages of 6 months and 6 years, and compared the patterns of PDGF receptor expression in these beta cells with that of islet beta cells from adults. They detected no PDGF receptor in the adult beta cells, but found it readily in the islet beta cells from young donors. Additional experiments showed that human beta cells function similar to the mouse beta cells in the study, and suggested that it may be possible to regulate human islet beta cell growth and insulin expression by manipulating PDGF receptor expression and activation.
"This work revealed that there are some pathways that haven't been explored in human beta cells that underlie the age-related loss of beta cell proliferation," said Kim. "This gives us a handhold onto a vaster problem: how to control human beta cell proliferation in a therapeutic way."
Continue Reading Below ↓↓↓
In addition to Kim and Chen, other Stanford researchers involved in the study include research assistant Xueying Gu; laboratory manager Yinghua Liu; senior researcher Jing Wang, MD; postdoctoral scholar Stacey Wirt, PhD; and associate professor of pediatrics and genetics Julien Sage, PhD.
The research was supported by the National Institutes of Health, the Stem Cell Network NRW, Deutsche Krebshilfe, the Dewey Family Fund, the Juvenile Diabetes Research Foundation, the Snyder Foundation, the Stinehart Foundation, the Larry L. Hillblom Foundation and the Howard Hughes Medical Institute.
Source: Stanford University Medical Center