June 2007 - University of Florida researchers have begun a clinical study of oral insulin to prevent or delay type 1 diabetes in people at risk for the disease.
The UF Health Science Center and Shands at UF are among 14 centers in the United States working with affiliate sites and participating physicians in Type 1 Diabetes TrialNet, a research group dedicated to the understanding, prevention and early treatment of type 1 diabetes.
"This is a unique opportunity to attempt to prevent the disease in relatives at risk for type 1 diabetes," said Desmond Schatz, M.D., medical director of UF's Diabetes Center of Excellence and principal investigator with the UF TrialNet Clinical Center. "The intervention may also offer hope for delaying the onset of the disease."
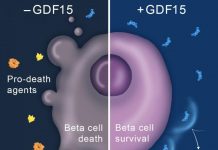
An estimated 1 to 2 million people with the disease have type 1 diabetes, which occurs when white blood cells vital to the body's defenses against infectious diseases attack insulin-producing beta cells in the pancreas. The insulin these cells produce regulates how the body uses and stores sugar and other food nutrients for energy.
Research has shown the pancreas is resilient and more than half its insulin-producing beta cells must be irreversibly destroyed before an individual develops symptoms of the disease, which can take months or even years to occur.
Continue Reading Below ↓↓↓
That long period prior to the onset of symptoms provides an opportunity for interventions aimed at preventing the disease's development, Schatz said.
In the study, University of Florida researchers are testing whether an insulin capsule taken by mouth once a day can prevent or delay type 1 diabetes in a specific group of people at risk for the disease.
An earlier trial suggested that oral insulin might delay type 1 diabetes for about four years in some people with islet cell autoantibodies in their blood. The presence of these autoantibodies alerts physicians to the destruction of insulin-producing cells up to 10 years before symptoms set in and indicates an individual is at greater risk of developing the disease.
For a person with high-risk genes and all three autoantibodies, the risk of developing diabetes in the next five years is greater than 50 percent, Schatz said.
"We hope that learning about the underlying immune events that set the stage for diabetes will help us identify ways to rein in the autoimmune attack on beta cells," he said.
Animal studies have also suggested that insulin taken orally might even prevent type 1 diabetes. Some scientists think that introducing insulin via the digestive tract induces tolerance, a quieting of the immune system.
First- and second-degree relatives of people with type 1 diabetes who may be at risk are initially being screened through TrialNet's natural history study, which is examining the immune and metabolic events that precede diabetes symptoms. Screening involves a simple blood test. Individuals enrolled in the natural history study are closely monitored for diabetes development and may be eligible to participate in the oral insulin trial or future studies that try to arrest the autoimmune process.
To lower blood sugar levels once diabetes occurs, patients need three or more insulin injections a day or treatment with an insulin pump. To prevent complications, they must regularly monitor their blood glucose, striving for a range that is as close to normal as possible.
Other diabetes studies under way at UF include a trial aimed at preserving insulin production in people recently diagnosed with type 1 diabetes, who often still have a supply of functioning beta cells. If these remaining beta cells can be protected with the help of insulin injections, more patients would be able to tightly control their blood glucose, preventing or delaying damage to the eyes, nerves, kidneys, heart and blood vessels.
Another TrialNet study seeks to turn off the body's attack on beta cells with rituximab, a monoclonal antibody that binds to and temporarily destroys a class of immune cells. The rituximab trial is recruiting patients with type 1 diabetes diagnosed within the previous three months. Also under way is a study testing whether a combination of two drugs approved by the FDA to prevent rejection after an organ transplant can slow or arrest the autoimmunity of type 1 diabetes.
Continue Reading Below ↓↓↓
Lastly, the Environmental Determinants of Diabetes in the Young, or TEDDY, study aims to discover the genes and environmental exposures that may cause type 1 diabetes through a newborn screening program. Babies found to be at a high risk of developing the disease may enroll in TEDDY II and will be tracked over time to examine environmental risk factors.
The Type 1 Diabetes TrialNet studies are funded by the National Institutes of Health. The Juvenile Diabetes Research Foundation International and the American Diabetes Association also support the initiative.
Source: University of Florida Health Science Center, Newswise