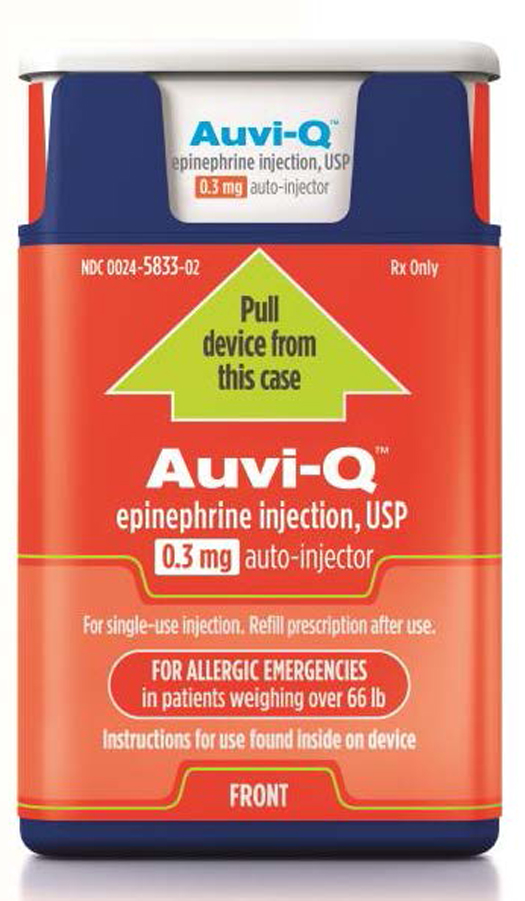
Sanofi US is voluntarily recalling all Auvi-Q because the products have been found to potentially have inaccurate dosage delivery. The recall involves all Auvi-Q currently on the market.
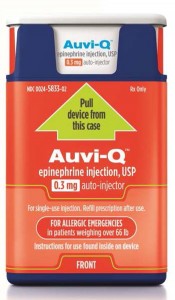
Sanofi US is voluntarily recalling all Auvi-Q® (epinephrine injection, USP). The recall involves all Auvi-Q currently on the market and includes both the 0.15 mg and 0.3 mg strengths for hospitals, retailers and consumers.
This includes lot number 2299596 through 3037230, which expire March 2016 through December 2016.
The products have been found to potentially have inaccurate dosage delivery.
Continue Reading Below ↓↓↓
If a patient experiencing a serious allergic reaction (i.e., anaphylaxis) did not receive the intended dose, there could be significant health consequences, including death because anaphylaxis is a potentially life-threatening condition.
As of October 26, 2015, Sanofi has received 26 reports of suspected device malfunctions in the US and Canada. None of these device malfunction reports have been confirmed. In these reports, patients have described symptoms of the underlying hypersensitivity reaction. No fatal outcomes have been reported among these cases.
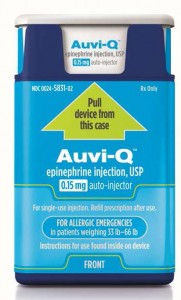
Auvi-Q (epinephrine injection, USP) is used to treat life-threatening allergic reactions (anaphylaxis) in people who are at risk for or have a history of these reactions. Auvi-Q is packaged with two active devices and one trainer device in a corrugate box. Auvi-Q was distributed throughout the United States via wholesalers, pharmacies and hospitals. All Auvi-Q is being recalled.
Sanofi US is notifying its distributors and customers who include doctors, pharmacies, wholesalers and other customers in the supply chain by letter, fax, email and phone calls and is arranging for return and reimbursement of all recalled products.
Customers with questions regarding this recall can go to www.Auvi-Q.com and call 1-866-726-6340 Monday through Friday 8 a.m. to 8 p.m. ET for information about how to return their Auvi-Q devices. Customers may also email [email protected]. Sanofi US will provide reimbursement for out of pocket costs incurred for the purchase of new epinephrine auto-injectors with proof of purchase.
Customers should immediately contact their healthcare provider (HCP) for a prescription for an alternate epinephrine auto-injector. In the event of a life-threatening allergic reaction (anaphylaxis), patients should only use their Auvi-Q device if another epinephrine auto-injector is not available, and then call 911 or local medical emergency services. Customers should contact their physician or HCP if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration. Sanofi US will continue to work closely with customers and regulatory authorities to resolve this issue in a timely manner.
Source: FDA
Continue Reading Below ↓↓↓