Can you really tell if a woman is at a higher risk of type 2 diabetes just by looking at her body shape? Researchers presented their findings at the American Society of Human Genetics annual meeting.
A genetic variant near the KLF14 gene regulates hundreds of genes that govern how and where women’s bodies store fat, which affects their risk of developing Type 2 diabetes, according to research presented at the American Society of Human Genetics (ASHG) 2015 Annual Meeting in Baltimore.
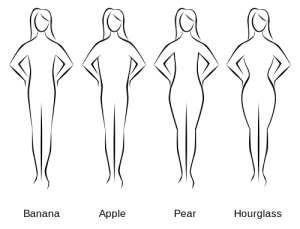
Specifically, different alleles, or versions, of the variant cause fat-storing cells to function differently. “At the whole-body level, these differences between alleles are not associated with changes to overall weight or body mass index, but they do affect women’s hip circumference,” explained Kerrin Small, PhD, Head of the Genomics of Regulatory Variation Research Group at King’s College London and lead author on the study.
“Previous studies have shown that on average, women who carry fat in their hips – those with a ‘pear-shaped’ body type – are significantly less likely to develop diabetes than those with smaller hips.
Looking at the variant we studied, large-scale genome-wide association studies show that women with one allele tend to have larger hips than women with the other one, which would have a protective effect against diabetes,” she said.
Continue Reading Below ↓↓↓
The variant is located near the KLF14 gene, which encodes a protein that Dr. Small and her colleagues discovered directly regulates the expression of hundreds of other genes in fat tissue. KLF14 is maternally imprinted, which means that a person’s expression of KLF14 and the resulting effects on fat tissue are determined by the version of the gene inherited from his or her mother; the father’s allele does not affect levels of this regulatory protein.
Researchers first identified the relationship between the variant near KLF14 and Type 2 diabetes risk in a large, genome-wide association study of a broad population. As with most studies of this type, the effect on diabetes risk was modest, though statistically significant. However, when Dr. Small and her colleagues focused on a more specific population, women who inherited the allele from their mothers, the effect size grew.

“These findings have important implications as we move toward more personalized approaches to disease detection and treatment,” Dr. Small said. “If we can identify the genes and protein products involved in diabetes risk, even for a subset of people, we may be able to develop effective treatment and prevention approaches tailored to people in that group.”
The researchers are currently exploring why the variant only seems to affect women. They have found that women have higher baseline levels of the KLF14 mRNA transcript, a precursor to the KLF14 protein, than men. This suggests the possibility of a threshold effect, in which men rarely or never attain the levels necessary to cause an increased risk of diabetes. Another hypothesis is that a different, sex-specific protein may interact with the KLF14 protein, enhancing or diminishing its effect in men or women.
To test these ideas, Dr. Small and her colleagues are investigating the specific mechanisms by which the variant near KLF14 affects KLF14 expression, as well as how the many genes regulated by KLF14 affect fat storage patterns and diabetes risk.
“Eventually, we hope to develop a comprehensive, predictive model of how genes affect risk of Type 2 diabetes in women,” she said.
Reference: Small K et al. (2015 Oct 10). Abstract: Adipose- and maternal-specific regulatory variants at KLF14 influence Type 2 Diabetes risk in women via a female-specific effect on adipocyte physiology and body composition. Presented at American Society of Human Genetics 2015 Annual Meeting. Baltimore, Md.
Source: American Society of Human Genetics