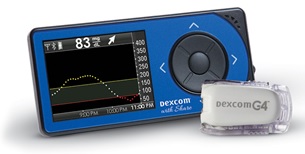
Dexcom Inc. is recalling it’s G4 Platinum and G5 Mobile continuous glucose monitoring system receivers due to an alarm failure. Use of these products can lead to serious injury or death.
The FDA has identified this as a Class I recall, the most serious type of recall. Relying on this device may cause serious injuries or death.
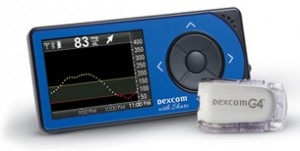
Recalled Devices
Name of device: Dexcom G4 PLATINUM Receiver, Dexcom G4 PLATINUM (Pediatric) Receive, Dexcom G4 PLATINUM (Professional) Receiver, Dexcom G4 PLATINUM Receiver with Share, Dexcom G4 PLATINUM (Pediatric) Receiver with Share, Dexcom G5 Mobile Receiver
- Model numbers: all models
- Lot numbers: all lots
- Manufacturing dates: July 29, 2011 to March 10, 2016
- Distribution dates: October 22, 2012 to March 10, 2016
- Devices recalled in the U.S.: 263,520 units nationwide
Device Use
Continue Reading Below ↓↓↓
The Dexcom Continuous Glucose Monitoring Systems are used to monitor the blood sugar (glucose) level of adult and pediatric patients with type 1 or type 2 diabetes.
These glucose monitoring systems include a sensor that is placed under the skin to measure blood glucose readings that are sent to a hand-held receiver.
They are used in combination with standard home glucose monitoring devices in the management of diabetes.


Reason for Recall
Dexcom Inc. is recalling the Continuous Glucose Monitoring Systems because the audible alarm may not activate in the receiver piece when low or high glucose levels (hypoglycemia or hyperglycemia) are detected.
Relying on this product for notification of low or high blood sugar could result in serious adverse consequences, including death as the auditory alarm may not sound and users might not be notified of low or high blood sugar.
Who May be Affected
- All patient groups using the Dexcom Continuous Glucose Monitoring Systems to monitor their blood glucose levels.
- Health care providers who prescribe these monitoring systems to patients in their care.
What to Do
On February 23, 2016, Dexcom Inc. sent an Important Customer Notification Letter with the following instructions to test the audio alert on the receiver:
- press the center button on your receiver to access the Main Menu
- scroll down to Profiles
- select Profiles
- scroll down to Try It
- select Try It
- scroll down to 55 Fixed Low
- select 55 Fixed Low
- verify that you receive vibrations first (vibratory portion of alarm), followed by beeps (audible portion of alarm).
Customers should contact Dexcom at their free hotline number: (844) 607-8398, if the audio alert does not work properly.
How do I report a problem?
Continue Reading Below ↓↓↓
Health care professionals and consumers may report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program either online, by regular mail or by FAX.