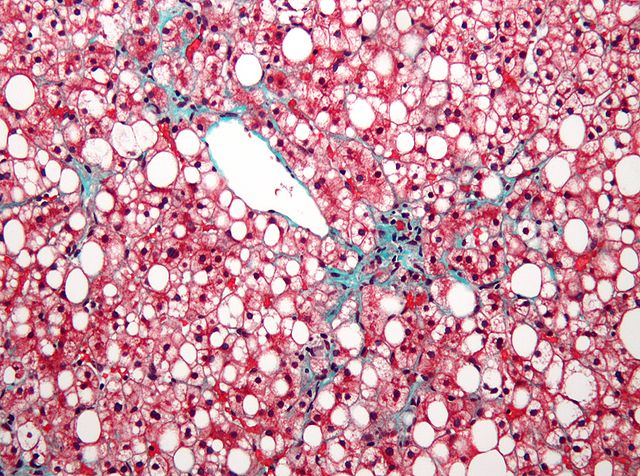
Promising initial results regarding sitagliptin, which is currently used to treat diabetes under the brand name Januvia, and it’s positive effect on liver disease risk were not confirmed by a recent controlled study.
New data presented today at The International Liver Congress 2016 in Barcelona, Spain, demonstrates that the drug sitagliptin (brand name: Januvia) – more commonly used to treat diabetes – was no more effective than placebo for reducing non-alcoholic fatty liver disease (NAFLD).
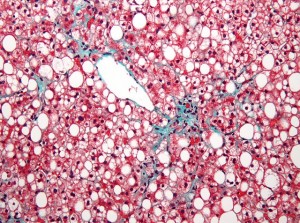
However, researchers did demonstrate that nonvasive imaging techniques based on Magnetic Resonance Imaging (MRI) were useful for measuring changes in liver fat and fibrosis, a finding that can inform future clinical trials in the disease.
NAFLD is the world’s most common liver disease and shares many features of metabolic syndrome, including obesity, insulin resistance and diabetes.1
Previous research had suggested that sitagliptin was able to improve some aspects of NAFLD, such as liver histology, but these studies had not tested the drug against a placebo control.
Continue Reading Below ↓↓↓
“We had hoped that results from some promising initial research around the use of sitagliptin in NAFLD could be confirmed in a randomised, double-blinded, placebo-controlled study,” says study author Dr Jeffrey Cui, MD, with the NAFLD Research Center, University of California, San Diego, USA. “NAFLD is a leading cause of liver disease in the Western world so new methods to reduce this disease burden are much needed.” The study’s lead author Dr Rohit Loomba, MD, Director, NAFLD Research Center, University of California, USA continues, “However, this trial does provide data on advanced MRI and MRE based measures to non-invasively assess treatment response, which would be very useful for the design of future trials.”
In the randomised, double-blind, allocation-concealed, placebo-controlled clinical trial of 50 American NAFLD patients with pre or early diabetes, patients were randomised to receive sitagliptin (100 mg/day orally) or placebo for 24 weeks.
The primary outcome was liver fat change (measured by MRI) in regions within each of the nine liver segments. Changes in hepatic fibrosis (scarring of the liver) were assessed using magnetic resonance elastography (MRE).
There was no significant difference in liver fat change in the group receiving sitagliptin (18.1% to 16.9%, p=0.2673) or the placebo group (16.6% to 14.0%, p=0.0729), and also no significant difference in mean liver fat change between the two groups (-1.3%, p=0.4096).
“Non-alcoholic fatty liver disease represents a significant problem worldwide. It is always disappointing when initial research cannot be replicated in a more rigorous trial setting, but we hope that the experiences shared of the imaging techniques employed in this study will help introduce novel, non-invasive imaging techniques into the clinical management of patients,” said Professor Frank Tacke, EASL Governing Board member.
Abstract: PS112, Sitagliptin versus placebo in the treatment of Nonalcoholic Fatty Liver Disease: A randomized controlled trial. This study was supported by an investigator initiated grant by Merck Inc
References
1 Gastaldelli A, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology. 2009;49:1537-1544.
Source: European Association for the Study of the Liver
Meeting: The International Liver CongressT 2016
Photo by Nephron / CC License