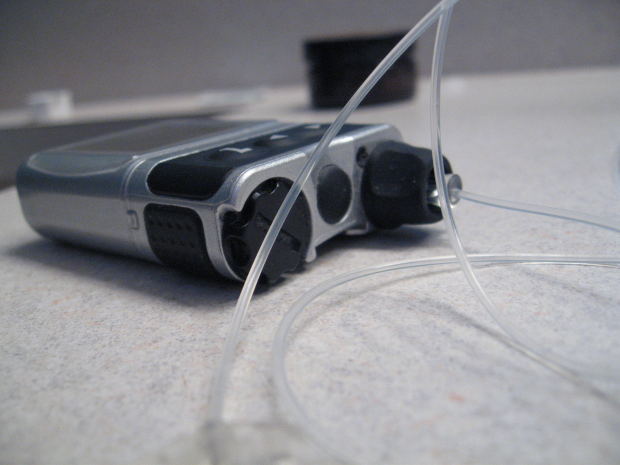
According to a joint statement by the American Diabetes Association and the European Association for the Study of Diabetes about the safety and efficacy of insulin pumps, a comprehensive safety overhaul is needed. The lead author further advises that anyone using a pump should have a pump failure plan.
Not enough is known about the safety and efficacy of insulin pumps, and a comprehensive safety overhaul – including greater access to data from pump manufacturers and public funding of research on the use of insulin pumps – is needed to allow health care teams to educate and support those using the devices, according to a joint statement by the American Diabetes Association and the European Association for the Study of Diabetes, published on March 16 and set to appear in the April issue of Diabetes Care.

The statement includes a list of recommendations intended to stimulate “the adoption of a more rigorous, standardized and transparent approach to safety.”
Currently, the U.S. Food and Drug Administration (FDA) conducts more rigorous reviews of devices such as pumps than the European Union. The statement therefore calls upon the FDA and EU to harmonize standards for pump manufacturing companies.
It also recommends providing a single, publicly accessible, international database for reporting adverse events, including both technical and human errors; requiring pump manufacturers to provide access to information, such as how many people use their products and the results of studies testing new features of pump design; and providing greater funding for independent clinical trials of “safety, efficacy, outcomes and adherence under real world conditions,” among other suggestions.
A full list of recommendations can be seen here.
“Technology is evolving rapidly for treating diabetes,” said Anne Peters, MD, Director, University of Southern California Clinical Diabetes Program and one of the lead authors on the statement. “While that’s certainly a good thing, we don’t have very good post-marketing surveillance for devices such as insulin pumps, particularly in Europe where manufacturers often introduce products prior to releasing them in the United States. We need to make sure we have sufficient data about how the devices are working once they hit the market, so that we can support patients by helping them understand how to prevent errors in using them.”
Continue Reading Below ↓↓↓
Roughly one million people use insulin pumps (continuous subcutaneous insulin infusion) worldwide, and more than 200 million people worldwide have been diagnosed with diabetes. Most people who use insulin pumps have type 1 diabetes, though some people with type 2 diabetes also use them.
Peters said the biggest problem her patients who use pumps run into is that the devices can break, and when they do it can take a few days to get a replacement. She advised that anyone using a pump should have a “pump failure plan” that includes maintaining a current printout of pump settings and having some long-acting insulin on hand.
Source: American Diabetes Association