Findings in human, mouse and zebrafish beta cells suggest a promising method to help treat all forms of diabetes, according to researchers at Joslin Diabetes Center.

More than a quarter of the 30 million people with diabetes in the United States depend on daily injections of insulin to maintain their blood glucose levels in a healthy range.
Scientists are investigating many techniques to help treat or potentially even cure many of these patients by increasing the body’s own insulin-producing pancreatic “beta” cells.
Now researchers at Joslin Diabetes Center have identified a key protein produced in the liver that aids in accelerating the growth of these cells.
“Making more functional beta cells is critical for treating all forms of diabetes,” says Rohit N. Kulkarni, M.D., Ph.D., Senior Investigator in the Section on Islet Cell and Regenerative Biology at Joslin Diabetes Center and Professor of Medicine at Harvard Medical School.
Continue Reading Below ↓↓↓
If his team is successful in using proteins that normally circulate in human blood as growth factors for beta cells, the approach may progress toward clinical trials more quickly than some potential therapeutic alternatives “because it takes away the uncertainty of what a foreign compound or a foreign molecule does to other tissues in the body,” Dr. Kulkarni says.
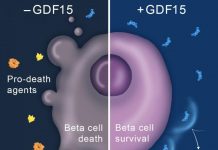
As the team reports today in the journal Cell Metabolism, a protein called serpinB1 can significantly boost beta cell growth in humans, mice and zebrafish. Mice that were genetically engineered to lack the protein also displayed diminished ability to grow beta cells.
Additionally, the researchers demonstrated that levels of serpinB1 in human blood are higher in people with insulin resistance, a condition that is a precursor to developing diabetes. (People with this condition may prevent developing diabetes by compensating and generating more beta cells, which are located in the clusters of pancreatic cells known as “islets”, due to the growth effects of serpinB1.)
Dr. Kulkarni, lead author Abdelfattah El Ouaamari, Ph.D., and their colleagues began their work in mice genetically modified to create insulin resistance in the liver. In an earlier study with this mouse model, which heightens the production of beta cells, they had discovered that proteins released from the liver and circulating in the blood were driving the increased proliferation of beta cells.
In the current study, the Joslin scientists worked with Wei-Jun Qian, Ph.D., of Pacific Northwest National Laboratories to analyze the proteins present in blood plasma from these mice and in the fluid surrounding their liver cells in culture. In both analyses, the researchers saw strikingly high levels of serpinB1. The serpinB1 gene was also highly expressed in liver cells.
Next, the group collaborated with Eileen Remold-O’Donnell, Ph.D., of Boston Children’s Hospital, who has a long-standing interest in serpins and whose group had built a synthetic version of the serpinB1 protein. When researchers incubated mouse or human islets with these proteins, they noticed a growth in beta cell proliferation.
The scientists knew that one of serpinB1’s roles is to inhibit activity of elastase , an enzyme that cuts one component of connective tissue. They identified two commercial elastase inhibitors and found that both compounds raised boosted beta cell production in mouse islets. One of the compounds also did so in human islets. Next, the researchers transplanted human islets into a mouse with a depleted immune system, along with a tiny pump that released the elastase inhibitor for two weeks. The human beta cells proliferated, as did the mouse’s own beta cells.
Further building up their evidence, the researchers experimented with mice that were genetically altered to remove the serpinB1 gene, exposing these mice to two measures that promote beta cell production in normal mice. In one experiment, the modified mice were put on a high-fat diet. In a second experiment, the mice were given a compound that raises blood glucose levels. In both cases, mice missing serpinB1 produced fewer beta cells than control mice.
Broadening the understanding of serpinB1 by examining its role in a very different organism, collaborators Olov Andersson and Christos Karampelias at Sweden’s Karolinska Institute made a model of zebrafish that overexpressed the serpinB1 gene. The result was a significant increase in the growth of beta cells.
Even more dramatically, when these fish were given a compound that destroyed all their beta cells, the Karolinska investigators could see new beta cells appearing. “This may have particular implications for treating type 1 diabetes, in which most of the beta cells are gone,” Dr. Kulkarni notes.
Continue Reading Below ↓↓↓
In humans, when Joslin scientists studied a group of 49 people they found a correlation between insulin resistance and the levels of serpinB1 circulating in the blood. Separately, they discovered that a family of Joslin patients has a mutation in the gene that is associated with type 2 diabetes, and they are following up to see if this mutation alters beta cell mass.
Dr. Kulkarni and his co-workers now are looking more broadly across various populations of people with diabetes both for serpinB1 mutations and for the levels of the protein in the blood. Additionally, the researchers are examining how the protein is regulated and the molecular mechanisms by which it boosts beta cell proliferation.
Importantly, beta cells in the presence of synthetic serpinB1 secrete insulin normally in response to blood glucose levels, suggesting that the protein promotes the growth of beta cells that are functioning normally, Dr. Kulkarni says. Using this method to grow the cells also avoids the questions about long-term stability that are encountered when developing beta-like cells by modifying stem cells or other types of cells, he points out.
Overall, identifying growth factors that naturally circulate in the blood offer potentially major advantages for building healthy beta cells, he says, “and we look forward to moving ahead rapidly with work to translate this research toward clinical use.”
Lead funders were the National Institutes of Health, JDRF and the JDRF/Sanofi Aventis Strategic Alliance. “A focus of JDRF’s Beta Cell Regeneration Program is understanding the mechanisms involved in the normal expansion of beta cells during times of increased insulin demand, with the aim of identifying safe and effective therapies to restore beta cell function in type 1 diabetes,” said Dr. Andrew Rakeman, Director of Discovery Research at JDRF. “These findings from Dr. Kulkarni and his colleagues are an exciting development towards realizing this goal for people with type 1 diabetes. We look forward to seeing how these findings might lead to the development of new therapies to aid insulin production leading to safe and normal blood-glucose levels.”
Other Joslin co-authors on the paper include Ercument Dirice, Nicholas Gedeon, Jiang Hu, Jun Shirakawa, Jeremie Boucher, Rachael Martinez, Dario De Jesus, Sevim Kahraman, Shweta Bhatt, Prapaporn Jungtrakoon, Allison Goldfine and Alessandro Doria. Other contributors include Eileen Remold-O’Donnell, Lifei Hou, Jessica Goodman and Yanping Gong of Boston Children’s Hospital; Wei-Jun Qian, Jian-Ying Zhou, Marina Gritsenko and Richard Smith of the Pacific Northwest National Laboratory; Olov Andersson and Christos Karampelias of the Karolinska Institute; Chong Wee Liew and Guifeng Qiang of the University of Illinois at Chicago; and Hans-Dietmar Beer of University Hospital Zurich.
Cell Metabolism, El Ouaamari et al.: “SerpinB1 Promotes Pancreatic β-Cell Proliferation” 10.1016/j.cmet.2015.12.001.
Source: Joslin Diabetes Center
Photo Credit: Joslin Diabetes Center
Journal: Cell Metabolism