Torrent Pharmaceuticals Limited is now voluntarily recalling ALL LOTS within expiry of Valsartan/Amlodipine/HCTZ, Valsartan/Amlodipine and Valsartan tablets to the consumer level.
This recall is due to the detection of trace amounts of an unexpected impurity found in an active pharmaceutical ingredient (API) manufactured by Zhejiang Huahai Pharmaceuticals.
The impurity detected in the API is N-nitrosodimethylamine (NDMA), which is a substance that occurs naturally in certain foods, drinking water, air pollution, and industrial processes, and has been classified as a probable human carcinogen as per International Agency for Research on Cancer (IARC) classification.
To date, Torrent Pharmaceuticals Limited has not received any reports of adverse events related to this recall.
Valsartan is used to control high blood pressure and for the treatment of heart failure. In combination with amlodipine or amlodipine plus hydrochlorothiazide, it is used to control high blood pressure.
Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication.
Continue Reading Below ↓↓↓
Patients who are on valsartan should continue taking their medication, as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment.
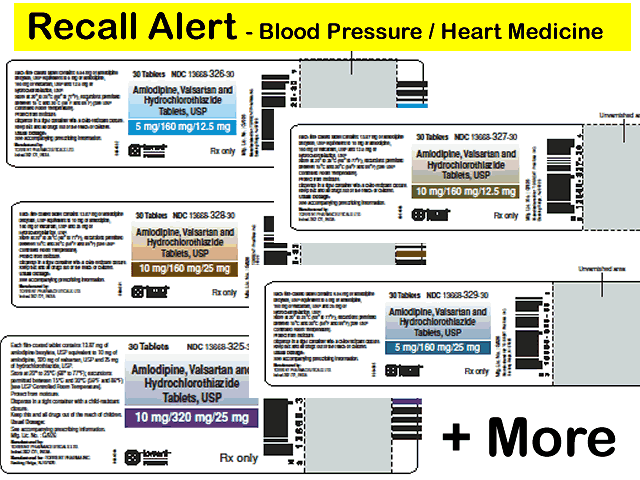
The products subject to recall are listed below and packaged in bottles. The product can be identified by checking the product name, manufacturer details and batch or lot number on the bottle containing these products.
| NDC | Product Description | Lot/Batch | Expiration Date |
|---|---|---|---|
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D025 | Nov-2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D026 | Nov-2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2E001 | Jan-2020 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2E002 | Jan-2020 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2E003 | Jan-2020 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2E004 | Jan-2020 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2E005 | Jan-2020 |
| NDC 13668-328-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/160mg/25mg, 30 Tablets | BBX9D004 | Nov-2019 |
| NDC 13668-328-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/160mg/25mg, 30 Tablets | BBX9E001 | Jan-2020 |
| NDC 13668-326-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/12.5mg, 30 Tablets | BBY1E001 | Dec-2019 |
| NDC 13668-326-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/12.5mg, 30 Tablets | BBY1E003 | Mar-2020 |
| NDC 13668-327-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/160mg/12.5mg, 30 Tablets | BBY2E001 | Mar-2020 |
| NDC 13668-329-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/25mg, 30 Tablets | BBY4D004 | Nov-2019 |
| NDC 13668-329-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/25mg, 30 Tablets | BBY4E001 | Jan-2020 |
| NDC 13668-329-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/25mg, 30 Tablets | BBY4E001 | Jan-2020 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D003 | Mar-2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D004 | Mar-2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D005 | Mar-2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D006 | Mar-2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D007 | Mar-2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D008 | Mar-2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D015 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D016 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D017 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D018 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D019 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D020 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D021 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D022 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D023 | Oct 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D024 | Nov 2019 |
| NDC 13668-328-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/160mg/25mg, 30 Tablets | BBX9D001 | Feb 2019 |
| NDC 13668-326-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/12.5mg, 30 Tablets | BBY1C002 | Sep 2018 |
| NDC 13668-326-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/12.5mg, 30 Tablets | BBY1E002 | Mar-2020 |
| 13668-327-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/160mg/12.5mg, 30 Tablets | BBY2D001 | Feb 2019 |
| 13668-327-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/160mg/12.5mg, 30 Tablets | BBY2D002 | Nov 2019 |
| NDC 13668-207-30 | Amlodipine and Valsartan Tablets 5 mg/160 mg, USP, 30 Tablets | BV53D004 | Oct 2019 |
| NDC 13668-206-30 | Amlodipine and Valsartan Tablets 10 mg/160 mg, USP, 30 Tablets | BV65D002 | Oct 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D013 | Oct 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84D010 | Oct 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84E001 | Dec 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D001 | Dec 2018 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D002 | Dec 2018 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D009 | Mar 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D010 | Apr 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D011 | Apr 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D012 | May 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D013 | May 2019 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2D014 | Aug 2019 |
| NDC 13668-328-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/160mg/25mg, 30 Tablets | BBX9D002 | Mar 2019 |
| NDC 13668-328-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/160mg/25mg, 30 Tablets | BBX9D003 | Jul 2019 |
| NDC 13668-326-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/12.5mg, 30 Tablets | BBY1D001 | May 2019 |
| NDC 13668-329-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/25mg, 30 Tablets | BBY4D001 | Apr 2019 |
| NDC 13668-329-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/25mg, 30 Tablets | BBY4D002 | Apr 2019 |
| NDC 13668-329-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 5mg/160mg/25mg, 30 Tablets | BBY4D003 | Jun 2019 |
| NDC 13668-068-90 | Valsartan Tablets, USP, 80 mg, 90 Tablets | BV46C007 | Sept 2018 |
| NDC 13668-068-90 | Valsartan Tablets, USP, 80 mg, 90 Tablets | BV46C008 | Oct 2018 |
| NDC 13668-068-90 | Valsartan Tablets, USP, 80 mg, 90 Tablets | BV46C009 | Oct 2018 |
| NDC 13668-068-90 | Valsartan Tablets, USP, 80 mg, 90 Tablets | BV46C010 | Oct 2018 |
| NDC 13668-068-90 | Valsartan Tablets, USP, 80 mg, 90 Tablets | BV46C011 | Nov 2018 |
| NDC 13668-068-90 | Valsartan Tablets, USP, 80 mg, 90 Tablets | BV46C012 | Nov 2018 |
| NDC 13668-069-90 | Valsartan Tablets, USP,160 mg, 90 Tablets | BV47C005 | Sep 2018 |
| NDC 13668-069-90 | Valsartan Tablets, USP, 160 mg, 90 Tablets | BV47C006 | Sept 2018 |
| NDC 13668-069-90 | Valsartan Tablets, USP, 160 mg, 90 Tablets | BV47D001 | Dec 2018 |
| NDC 13668-070-90 | Valsartan Tablets, USP, 320 mg, 90 Tablets | BV48D001 | Dec 2018 |
| NDC 13668-070-90 | Valsartan Tablets, USP, 320 mg, 90 Tablets | BV48D002 | Dec 2018 |
| NDC 13668-207-30 | Amlodipine and Valsartan Tablets 5 mg/160 mg, USP, 30 Tablets | BV53C006 | Nov 2018 |
| NDC 13668-207-30 | Amlodipine and Valsartan Tablets 5 mg/160 mg, USP, 30 Tablets | BV53D001 | Feb 2019 |
| NDC 13668-207-30 | Amlodipine and Valsartan Tablets 5 mg/160 mg, USP, 30 Tablets | BV53D002 | Feb 2019 |
| NDC 13668-207-30 | Amlodipine and Valsartan Tablets 5 mg/160 mg, USP, 30 Tablets | BV53D003 | Sep 2019 |
| NDC 13668-206-30 | Amlodipine and Valsartan Tablets 10 mg/160 mg, USP, 30 Tablets | BV65C002 | Sep 2018 |
| NDC 13668-206-30 | Amlodipine and Valsartan Tablets 10 mg/160 mg, USP, 30 Tablets | BV65C003 | Oct 2018 |
| NDC 13668-206-30 | Amlodipine and Valsartan Tablets 10 mg/160 mg, USP, 30 Tablets | BV65C004 | Nov 2018 |
| NDC 13668-206-30 | Amlodipine and Valsartan Tablets 10 mg/160 mg, USP, 30 Tablets | BV65D001 | Aug 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77C011 | Oct 2018 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D001 | Feb 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D002 | Feb 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D003 | Feb 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D004 | Feb 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D005 | Feb 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D006 | Feb 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D007 | Feb 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D008 | May 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D009 | Aug 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D010 | Sep 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D011 | Sep 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77D012 | Sep 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84C011 | Oct 2018 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84D001 | Jan 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84D002 | Jan 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84D005 | Feb 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84D006 | Feb 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84D007 | Feb 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84D008 | May 2019 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84D009 | May 2019 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77C009 | Aug 2018 |
| NDC 13668-204-30 | Amlodipine and Valsartan Tablets 10 mg/320 mg, USP, 30 Tablets | BV77C010 | Aug 2018 |
| NDC 13668-207-30 | Amlodipine and Valsartan Tablets 5 mg/160 mg, USP, 30 Tablets | BV53C004 | Aug 2018 |
| NDC 13668-207-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV53C005 | Aug 2018 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84C006 | Aug 2018 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84C007 | Aug 2018 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84C008 | Aug 2018 |
| NDC 13668-205-30 | Amlodipine and Valsartan Tablets 5 mg/320 mg, USP, 30 Tablets | BV84C009 | Aug 2018 |
| NDC 13668-325-30 | Amlodipine, Valsartan and Hydrochlorothiazide Tablets, USP 10mg/320mg/25mg, 30 Tablets | BBX2C007 | Aug 2018 |
| NDC 13668-069-90 | Valsartan Tablets USP, 160 mg, 90 Tablets | BV47C003 | Aug 2018 |
| NDC 13668-069-90 | Valsartan Tablets USP, 160 mg, 90 Tablets | BV47C004 | Aug 2018 |
| NDC 13668-068-90 | Valsartan Tablets USP, 80 mg, 90 Tablets | BV46C003 | Aug 2018 |
| NDC 13668-068-90 | Valsartan Tablets USP, 80 mg, 90 Tablets | BV46C006 | Aug 2018 |
Product and Packaging Photos






Continue Reading Below ↓↓↓


















Valsartan/Amlodipine/HCTZ. Valsartan/Amlodipine and Valsartan tablets were distributed Nationwide to Torrent’s wholesale, distributor, repackager and retail customers. Torrent Pharmaceuticals Limited is notifying its distributors and customers by phone and in writing to immediately discontinue distribution of the specific lots being recalled and to notify their sub-accounts.
Torrent is arranging for return of all recalled products to Qualanex. Instructions for returning recalled products are given in the recall letter.
Consumers with medical questions regarding this recall or to report an adverse event can contact Torrent Pharmaceuticals Limited at:
1-800-912-9561 (live calls received 8:00 am – 5:00 pm Eastern Time, voicemail available 24 hours/day, 7 days/week).
Email: [email protected]
Consumers should also contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Any general questions regarding the return of this product please contact Qualanex at 1-888-424-4340 or 1-800- 505-9291 (live calls received 8 am -5:30 pm Eastern Time).
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.