Researchers have found a sensor for the reactive molecules linked to diabetic complications using worms, which may provide new therapies for serious diabetes health problems like neuropathy.
Researchers at the Buck Institute have found a sensor for the reactive molecules linked to diabetic complications, providing a pathway to study many of the ravages of a disease that affects 29 million people in the US.
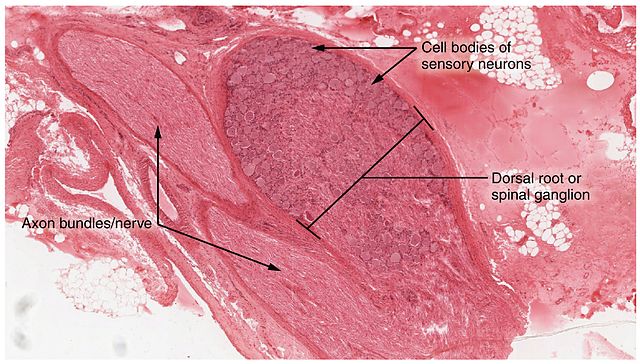
The work, done in the nematode C. elegans and detailed in Current Biology, provides particular promise for those suffering from painful diabetes-related nerve damage.
Scientists identified two natural compounds, including the supplement alpha-lipoic acid, which prevented nerve damage in worms experiencing a similar hypersensitivity to touch as do humans who suffer from diabetic neuropathy. The compounds essentially cured the nematodes of the condition.
“We realize that it is a huge leap between humans and C. elegans, but it’s important to note that the pathway involved in neuropathy is conserved among species,” said Buck professor Pankaj Kapahi, PhD, senior scientist on the study. “We now have a good model and a novel pathway that allows us to study many of the complications of diabetes. We also have novel compounds that slow the accumulation of these toxic molecules and show real promise in alleviating a very painful condition.”
Continue Reading Below ↓↓↓
The work highlights one of the goals in the Kapahi lab – to develop treatments that address the life-threatening complications of type 2 diabetes, which include neurodegeneration, cardiovascular diseases, and kidney failure. The aging of the population highlights the need for therapies, given that in the US nearly 27 percent of all adults 65 or over are afflicted with diabetes.
This research focused on identifying the root cause for diabetic neuropathy, a dysfunction of the peripheral nerves, which can cause numbness, weakness, and pain. The American Diabetes Association says about half of all people with diabetes have some form of nerve damage.
Zeroing in on AGEs
The study, led by postdoctoral fellows Jyotiska Chaudhuri, PhD, and Neelanjan Bose, PhD, focused on a compound called Methylglyoxal (MGO) which is formed from glucose in the body. MGO is extremely toxic and reacts with essential proteins, DNA, and lipids to form AGEs- advanced glycation end products which have been implicated as the cause of many diabetic complications. AGEs are also suspected of contributing to Alzheimer’s and Parkinson’s diseases, which have been linked to type 2 diabetes. Kapahi says those who want to see AGEs form in real time just need to put some meat in a hot skillet. “The browning that you see when you sear meat is an example of the AGEs-related cross-linking that occurs in proteins,” he said.
AGEs affect nearly every cell type and are a normal byproduct of metabolism and are not generally a problem for those who eat a healthy diet. But the production of AGEs ramps up when blood sugar is out of control, as it is in diabetes. Kapahi says that, at some point, damage from accumulated AGEs becomes irreversible. “Our goal is to stop them from forming in the first place,” he added.
TRPA1 and methylglyoxal
In the lab Chaudhuri and Bose identified a critical sensor for MGO, a protein called TRPA1 (and a similarly named pathway) that responds to high MGO in the body and detoxifies them. “TRP (Transient Receptor Potential) ion channels are evolutionarily conserved proteins that function in sensing many stimuli,” said Chaudhuri. “Of them, TRPA1 is a well-known mechanosensory receptor that responds to many noxious stimuli – including pain. Because our model exhibited mechanosensory phenotypes we examined if it played a role in diabetic neuropathy.” The postdocs boosted the activity of TRPA1 by feeding the nematodes alpha-lipoic acid and podocarpic acid, a compound found in the bark of a conifer that grows in Australia and New Zealand. Bose said the results were dramatic. “The worms were no longer hypersensitive to touch, they moved normally, they exhibited no neuronal damage and lived a long healthy life. Our work demonstrates that TRPA1 activity is critical in limiting diabetic complications.”
Alpha-lipoic acid is an antioxidant naturally found in organ meats such as liver and kidney as well as in yeast, spinach and broccoli. It’s also available as a supplement. Several human studies suggest that alpha-lipoic acid helps lower blood sugar levels. It has been used for years to treat the symptoms of peripheral diabetic neuropathy in Germany, where it is most commonly administered in high doses intravenously.
The larger picture
Kapahi hopes the new model, with its focus onTRPA-1, MGO and AGEs, will help researchers understand and deal with the complex pathology of type 2 diabetes and its complications. Noting the variability of responses among those with the disease, Kapahi said, “Some patients with diabetes get complications, others don’t. Some with pre-diabetes develop problems. Why do some get diabetic retinopathy and others develop kidney problems? There are likely genetic risk factors at play and focusing solely on controlling blood glucose won’t be sufficient to deal with patient needs.” Kapahi added that the short-lived worm provides a way to get to the root cause of diabetic complications, and also facilitates rapid drug discovery. “It can take decades for symptoms to develop in humans – in the worm we see problems within two weeks.”
Continue Reading Below ↓↓↓
He also thinks the findings could help hone in on research on substances that are known to activate TRPA-1, such as cinnamon, garlic, and wasabi. “There is some evidence that people who eat spicy food are protected against diabetes,” said Kapahi. “Maybe it’s because of TRPA-1.”
Researchers in the Kapahi lab will next focus on AGEs and their implications for Alzheimer’s and Parkinson’s diseases. They want to see if they can rescue mice who suffer from conditions increasingly linked to type 2 diabetes.
Citation: A Caenorhabditis elegans model elucidates a conserved role for TRPA1-Nrf signaling in reactive alpha-dicarbonyl detoxification
DOI: 10.1016/j.cub.2016.09.024
Other Buck researchers involved in the study include David Hall, Alexander Rifkind, Dipa Bhaumik, T. Harshani Peiris, Manish Chamoli, Catherine H. Le, Gordon J. Lithgow, and Arvind Ramanathan. Other collaborators include Jianke Gong and Jiafeng Liu from the College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, Hubei, China and X.Z. Shawn Xu from the Life Sciences Institute and Department of Molecular and Integrative Physiology, University of Michigan, Ann Arbor.
Acknowledgements:
This work was supported by grants from the American Federation of Aging Research (to P.K.), Larry L. Hillblom Foundation (to P.K.), and the NIH (T32 AG000266 to J.C.; R01AG038688, AG038012, AG045835 to P.K.; andR01AG048072 to X.Z.S.X.) and the Buck Institute Impact Circle.
Source: Buck Institute / Related Journal Article